A Ray of Hope? Breast Cancer Vaccine Overview and Expert Opinions
After two decades of research, a promising breast cancer vaccine has passed its first human trials. Health Reporter decided to take a closer look at this development and investigate whether or not it meets the expectations.

Lately, the @feminist Instagram account has taken the internet by storm with its groundbreaking post. It was about a promising breast cancer vaccine that has passed human trials after two decades of research.
As this news continues to spread across social media platforms, Health Reporter decided to take a closer look at this development and investigate whether or not it meets the expectations.
Breast cancer accounts for one-third of all cancer cases in women and results in the deaths of 43,000 people each year.1 Approximately one in eight women will develop breast cancer during their lifetime.2
More than two million new cases are registered annually worldwide.3 About 30% of patients are in an advanced stage.4 Although it is treated, the survival rate is incredibly low.
In the US, breast cancer rates have been stable for several decades. Yet, in the 2000s, they began to increase by about 0.5% annually.5
With a promising vaccine, the end of breast cancer could be on the horizon.
But is it too good to be true? Health Reporter found out the unspoken details of this revolutionary vaccine. Also, we asked doctors about the potential and risks it holds for breast cancer treatment.
The Latest on the Breast Cancer Vaccine Discovery in a Nutshell
An experimental breast cancer vaccine showed promise in November 2022.6 It safely generated a robust immune response to HER2, a key protein in breast tumors. Elevated levels of HER2 are responsible for 30% of all breast cancer cases.7
While the study is still in its early stages, the results are exciting enough to warrant further testing. This plasmid DNA vaccine will be evaluated in a larger, randomized clinical trial to confirm its effectiveness.
What Is This Breast Cancer Vaccine?
Cancer vaccines existed before, such as HPV vaccines. They prevent cervical cancer by targeting tumor-causing strains of human papillomaviruses (HPV).
Yet, nowadays, scientists focus on training the body to recognize tumor cells rather than viruses. Patients are given immunization with vaccines to reduce the risk of cancer.
What sets vaccines apart from radiation and chemotherapy? Vaccines are a more specific way to target cancer cells, unlike radiation and chemotherapy, which also harm healthy cells. It can cause side effects such as hair loss, infections, and mouth sores.
Some breast cancers, called HER2-positive, grow faster and are more likely to return after treatment. This includes people who have a higher risk of developing cancer. For example, those with BRCA1 or BRCA2 mutations or those who have had cancer and are at risk for recurrence.
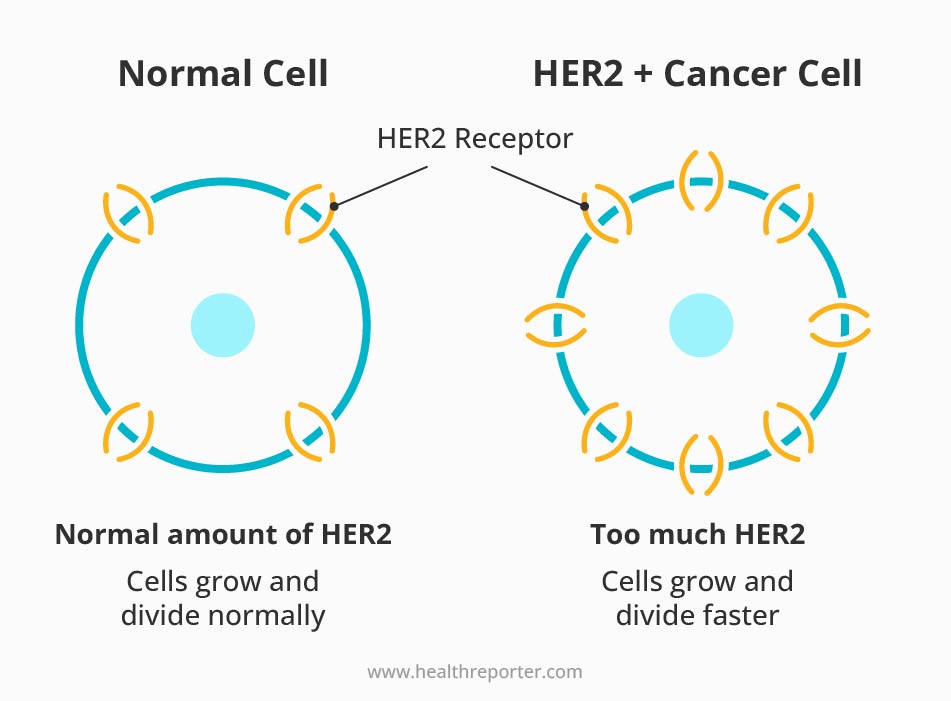
One of the most promising vaccines against HER2-positive cancers is the plasmid DNA vaccine. It uses DNA to tell cells to make a specific piece of the HER2 protein. This process triggers an immune response and helps the body find and destroy certain tumors.
Plasmid DNA vaccine prevents recurrence, not primary breast cancer.
Scientists have been testing this vaccine for 20 years on 66 patients with advanced HER2-positive breast cancer.8 The trial was done in three different dose levels, and the main goal was to see if the vaccine was safe.
The researchers also checked on patients for 10 years to ensure no problems with the immune system attacking healthy cells. This vaccine was found to be very safe. It only caused side effects as:
- Redness and swelling at the injection site
- Some fever, chills, and flu-like symptoms
The trial was not designed to see how well the vaccine worked against breast cancer. Although, early results showed that 80% of the patients survived the 10-year follow-up. It is much better than the typical survival rate for advanced HER2-positive breast cancer patients.
Why Is the Road to a Cancer Vaccine Longer Than to COVID-19 Vaccines?
Many people on social media compared the breast cancer vaccine to a COVID-19 vaccine.9 The former took 20 years to pass the first stage of human clinical trials. The latter was made available to the public less than a year after development began.
Why is there such a stark contrast in development timelines? Dr. Edna Skopljak, the Health Reporter medical expert, sought to answer this question:
“Developing a vaccine for cancer is far more complex than that for a virus. Unlike a virus, cancer is caused not by a single pathogen but by a variety of genetic mutations and environmental factors.
Viruses enter our body from the outside, while cancer is our mutated cells. And creating a vaccine that helps the body fight itself is a much longer process.
Cancer cells vary between individuals, making it challenging to create a one-size-fits-all treatment. Furthermore, cancer cells evolve and change over time. So, scientists must develop a vaccine that is effective against all potential mutations.
Most importantly, the clinical trial process for cancer vaccines is more complex. They often require larger sample sizes and longer follow-up periods.”
Another expert has given their support to this viewpoint. Dr. Sony Sherpa, a holistic physician from Nature’s Rise, said:
“It is true that the COVID-19 vaccine was developed at a record speed within one year. Yet, its timeline cannot be compared to the timeline required for breast cancer vaccine development.
Breast cancer doesn’t have a single cause. Its cells are made up of different types and mutations that vary by person.
So, it is difficult to identify any singular targetable protein or antigen on which vaccines can be designed, tested in clinical trials, and finally approved by regulatory bodies like the FDA.
Developing an effective vaccine against breast cancer requires multi-dimensional analysis. Not just purely focusing on the differences between viral diseases and cancers.”
Phases of cancer clinical trials, explained simply
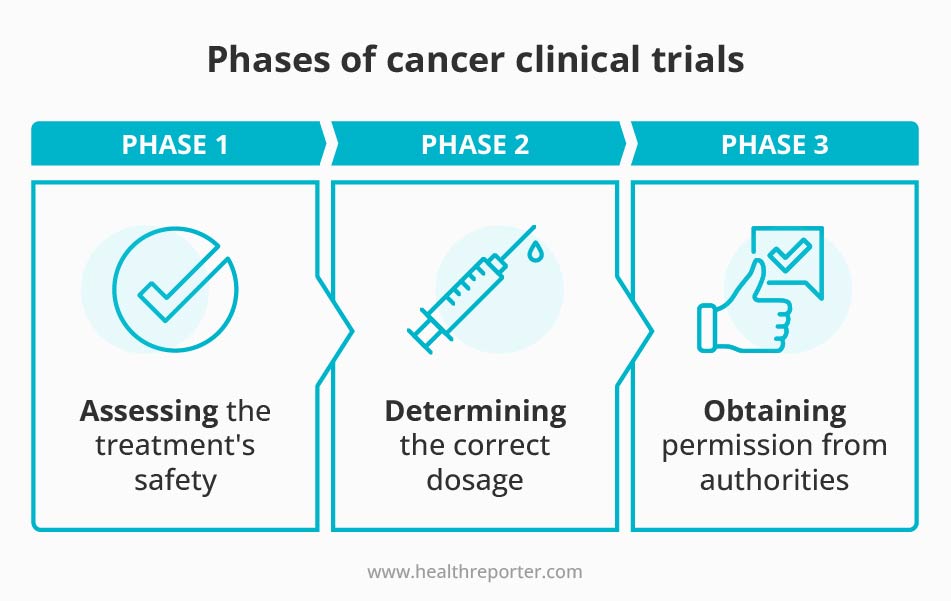
Clinical trials are a crucial step in developing new cancer treatments. They are often divided into three phases, with each stage serving a specific purpose in the overall process.10
The first phase, known as Phase 1, focuses on assessing the new treatment’s safety. This is typically done with a few people over a relatively short time frame. Phase 1 aims to determine whether the new treatment is safe and to identify side effects.
In Phase 2, the focus shifts to determining the correct dosage of the treatment. This phase is also conducted with a small number of people. The goal is to find the optimal dose of the medicine that will be most effective while minimizing the risk of side effects.
Once the safety and dosage have been established, the treatment moves to Phase 3. This phase is the largest and most comprehensive.
It involves comparing the new treatment to currently available therapies for cancer. Phase 3 can take several years to complete and involves many patients. The goal is to determine whether the new treatment is more effective and has a better side effect profile than the existing treatments.
Once the Phase 3 clinical trials are complete, the information is used to determine if the treatment can be used for patients. This information is presented to authorities, funding agencies, and governments. They obtain approval for the treatment to be used in the population.
Currently, the plasmid DNA vaccine is in a new Phase 2 trial with a larger group of patients. Preliminary results are expected in the next couple of years.
How soon will this breast cancer vaccine be widely available? Some experts predict it can be possible by 2030.11
It’s important to note that some MDs take a skeptical stance on both COVID-19 and breast cancer vaccines. Dr. Jennifer Simmons, a breast surgeon with 20 years of experience, said:
“The technology for the COVID-19 vaccine was around for 10 years previously. Because the mRNA vaccine already existed, they could quickly adapt it to code for the spike protein. In the time of the original variant, where coronavirus was a threat to life for those with chronic illness, its emergency use could be justified.
However, the variants have become less and less virulent and now are little more than the equivalent of the common cold.
I share the same concern over both these vaccines. Breast cancer and death from COVID-19 share a common etymology. They are more prevalent and dangerous in people with pre-existing metabolic diseases.
Overweight, obese, hypertensive, diabetic, autoimmune – all these conditions are associated with a chronic inflammatory state. I wish we would direct our resources towards developing these costly drugs toward teaching people to be healthy and to lead an anti-inflammatory life.
An ounce of prevention is worth a pound of cure. Breast cancer is an environmental disease. Instead of sterilizing the person, maybe we should clean up our environment?”
The MD’s Final Weigh in
The medical community’s opinion on breast cancer vaccines varies. While some researchers and oncologists believe that vaccines have the potential to be a powerful tool in the fight against breast cancer, others are more cautious and believe more research is needed before a vaccine can be widely implemented.
Dr. Edna Skopljak, the Health Reporter medical expert, said:
“I am very impressed with the latest development of the trial. One of the problems with breast cancer treatment is disease recurrence because a small amount of cancer cells remain undetected. This could provide an additional tool for breast cancer treatment to eliminate remaining hidden cancer cells.
At this stage, it could boost the industry to invest more in cancer treatments focusing on increasing patients’ immune systems to fight against cancer cells. The drive will significantly shift towards targeted therapies. They are designed to treat only the cancer cells and minimize damage to normal, healthy cells.
Like every new treatment, more research is needed to understand the long-term benefits and potential side effects.
If, after 5 years, this vaccine will be available for breast cancer treatment and after 10 years, it will replace all previously existing breast cancer treatments, I would see one concern.
People may lose interest in caring for their overall health, eating habits, and environment. Because why bother with maintaining a healthy lifestyle when you can inject a vaccine?”
On the other hand, certain medical doctors exhibit a more cautious demeanor with similar conclusions. Dr. Jennifer Simmons, a breast surgeon with 20 years of experience, said:
“I am not sure we should be excited about this quite yet. My biggest concern for this particular vaccine is that there are normal cells in the body that have HER2 receptors on them, like cardiac cells.
I worry about the vaccine’s effect on normal tissue and creating autoimmune disease. And it is roadblocks like this that have delayed development.
However, I appreciate the pace at which they are working because I believe it is being done out of respect for the first principle of medicine – do no harm.
We don’t want to make the solution worse than the problem. If this vaccine prevents people from dying of breast cancer but gives them heart disease, have we helped them?
I am not convinced that this is the revolutionary approach it is touted to be. Cancer is a heterogeneous disease. There are countless potential targets. We certainly can’t develop a vaccine for each one.
Ultimately, this will not be the solution. The solution is in us. In living healthy lives that don’t promote disease.”
Closing Remarks
Every day, women across the world are faced with the daunting reality of breast cancer. However, despite its devastating impact, hope persists.
With medical advancements making leaps and bounds, more and more women can lead fulfilling lives after treatment. The 5-year relative survival rate for breast cancer is now around 90%, up from 75% in the 1970s.12
Though the road to recovery may be tough, there are still ways to manage the disease. Joining clinical trials for cutting-edge treatments can provide a glimmer of hope and the chance to be a part of something bigger. It’s always a personal decision, and trusted resources like clinicaltrials.gov can help find the right one.
*This material is free from sponsored influence and represents the personal opinions of our experts.
Sources
- The Race to Make a Vaccine for Breast Cancer:
https://time.com/6220114/breast-cancer-vaccine-development/ - Breast Cancer Statistics: How Common Is Breast Cancer?
https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html - Cancer – WHO:
https://www.who.int/news-room/fact-sheets/detail/cancer - Cancer – WHO:
https://www.who.int/news-room/fact-sheets/detail/cancer - The Race to Make a Vaccine for Breast Cancer:
https://time.com/6220114/breast-cancer-vaccine-development/ - Plasmid DNA Vaccine Encoding ERBB2 Intracellular Domain in Patients With Advanced ERBB2+ Breast Cancer:
https://jamanetwork.com/journals/jamaoncology/article-abstract/2797975 - Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170925/ - Plasmid DNA Vaccine Encoding ERBB2 Intracellular Domain in Patients With Advanced ERBB2+ Breast Cancer:
https://jamanetwork.com/journals/jamaoncology/article-abstract/2797975 - Source:
https://www.instagram.com/p/Cnzo99sP0hY/ - Phases of clinical trials:
https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/what-clinical-trials-are/phases-of-clinical-trials - Could the Dream of a Cancer Vaccine Become Reality? Why Covid-19 Shots Brought Us One Step Closer
https://katiecouric.com/health/cancer/cancer-vaccine-biontech-cofounders/ - Survival Rates for Breast Cancer:
https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
















































 Select your language:
Select your language: 








